
“The BDI will translate new and existing technologies to practical health solutions and products, and vitally, maximise their accessibility to vulnerable populations.”
The Need for Rapid Point-Of-Care Diagnostics
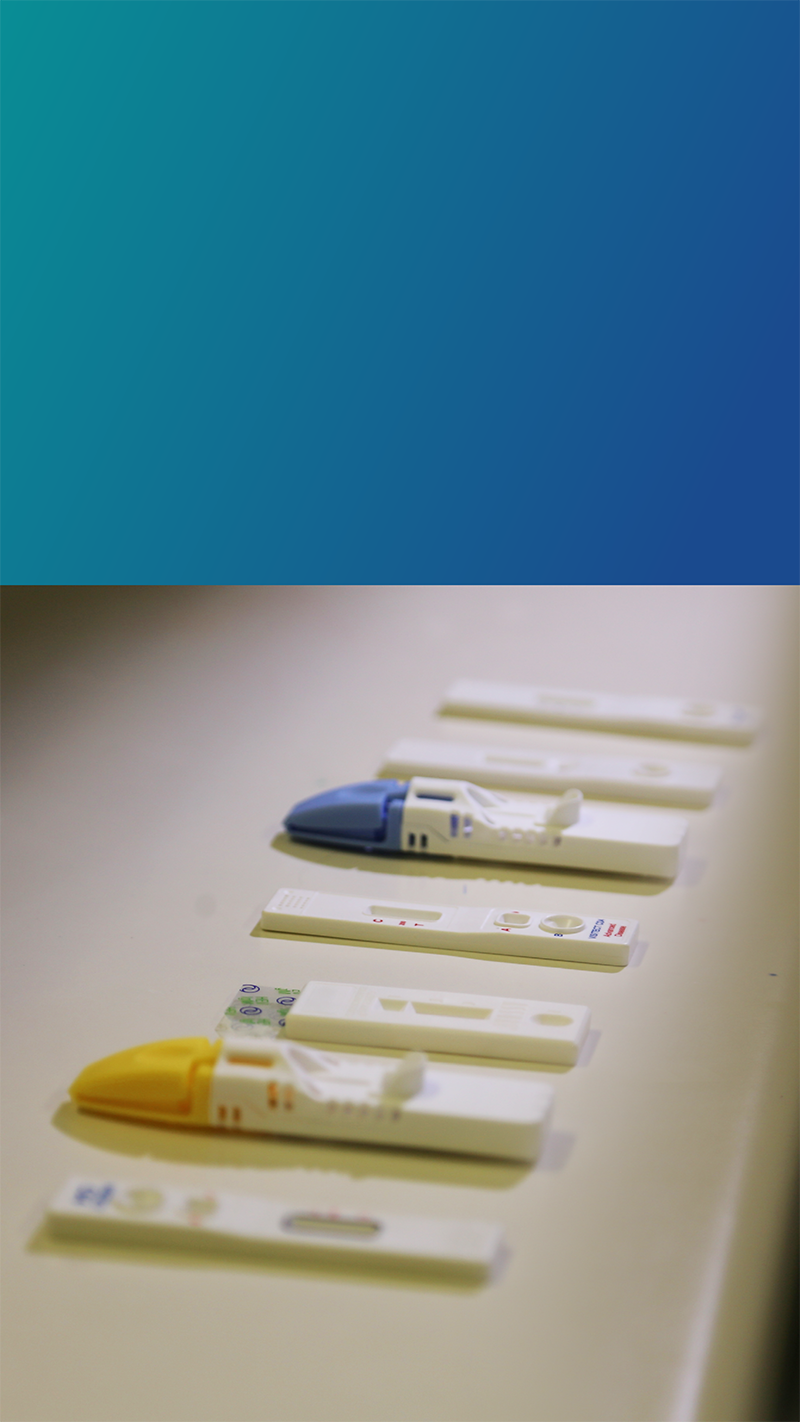
Diagnostics play a critical role in increasing access to quality treatment for those in the developing world, particularly for infectious diseases, such as hepatitis and HIV.
Unfortunately, the majority of those individuals living in rural and remote areas of the developing world have limited access to testing. This is mainly due to logistics, training and equipment challenges associated with many tests in use requiring routine operations in centralised laboratories.
There is a clear need for the development of simple, low-cost and accurate tests that can be operated without associated infrastructure, in the field.
Developing diagnostics that can be easily and effectively operated in remote and rural areas of the developing world is essential to improving the health of poor and vulnerable communities globally.
The BDI is working to fill that gap through the development of rapid point-of-care diagnostics to maximise accessibility.
Our Track Record
Over the last 15 years, Burnet has
developed a number of immunoassay
products, particularly point-of-care (POC)
assays but also lab-based (ELISA) tests
focused on unmet health needs.

Our Track Record
Over the last 15 years, Burnet has developed a number of immunoassay
products, particularly point-of-care (POC) assays but also lab-based (ELISA) tests focused on unmet health needs.
Current Development Work
Disease Elimination
- COVID-19 diagnosis
- COVID-19 neutralising antibody screening
- Hepatitis diagnosis
- Liver function screening
- Syphilis diagnosis
Health Security and Pandemic Preparedness
- COVID-19
- Zoonotic respiratory infections
- Measles
Maternal, Child and Adolescent Health
- Early infant HIV
- Anaemia
- Neonatal syphilis
- Neonatal sepsis
BDI Consulting
View consulting services >

BDI Consulting
Clinical + Analytical
Performance Studies for IVDs
Scientific Advice
View consulting services >


Explore our sustainable model for diagnostic development


The Burnet Diagnostics Initiative (BDI) is an initiative focussed on the development of new diagnostic products under a quality management system and in line with the best in industry practice. The Commercialisation and Research Translation (CRT) team supports the BDI with commercial and research translation expertise.

















